Cambrian ecosystems
This session is a workshop exploring Cambrian ecosystems, designed to help you to revise concepts from earlier in the course, and guide your approach to the Wenlock assignment.
It's a little different to previous sessions: the focus is on developing your interpretation and critical thinking skills, so we'll be working through the content in groups. The online material duplicates what we will cover in the classroom, but is provided here to allow you to spend additional time grappling with the concepts – and pondering how they might be applied to your Wenlock project.
As such there's no explicit preparation for the session, but you may wish to watch the refresher on the Cambrian Explosion (embedded below). Perhaps you could use the time gained to progress your Wenlock data analysis.
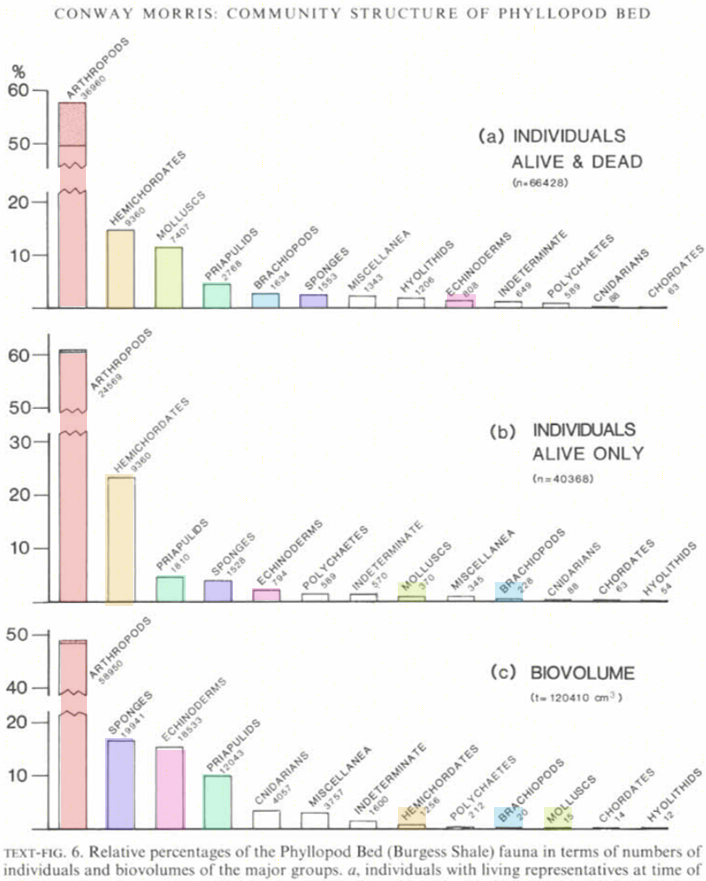
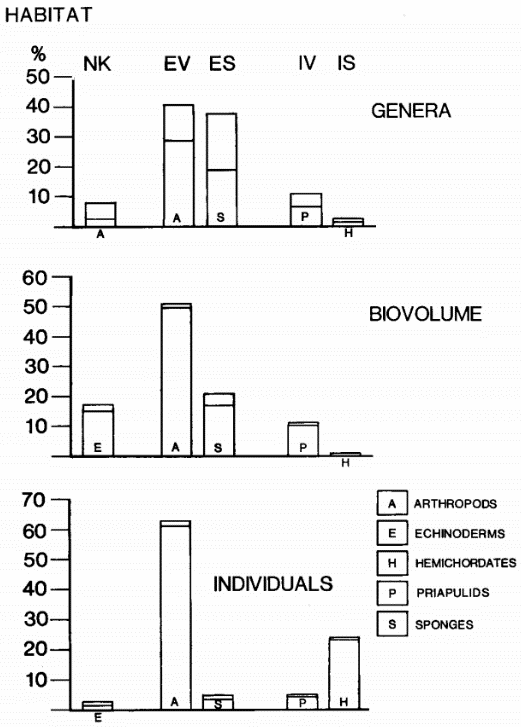
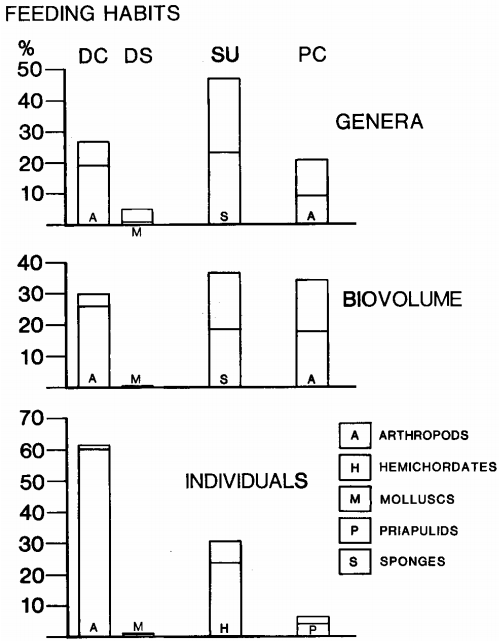
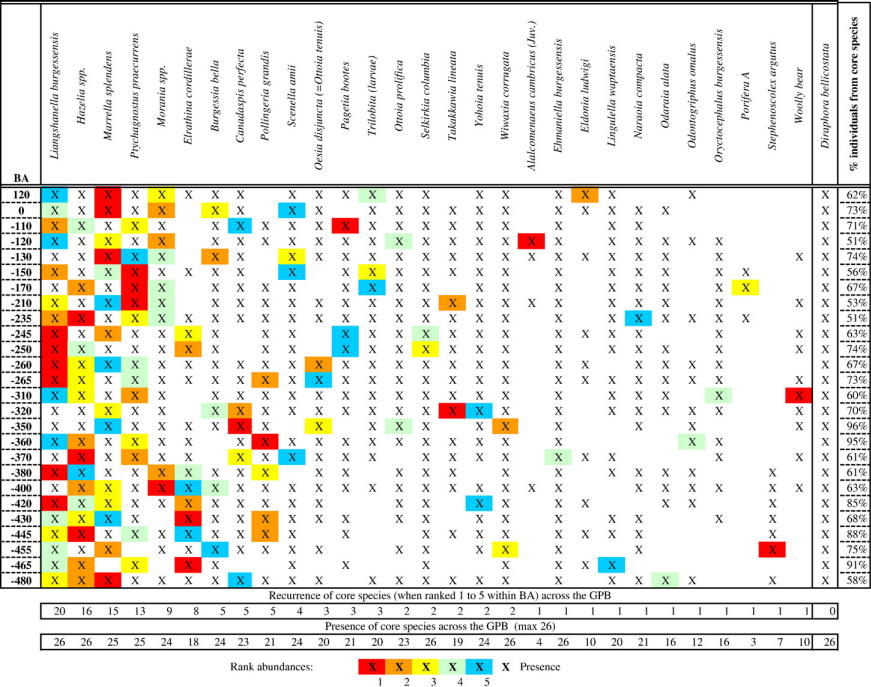
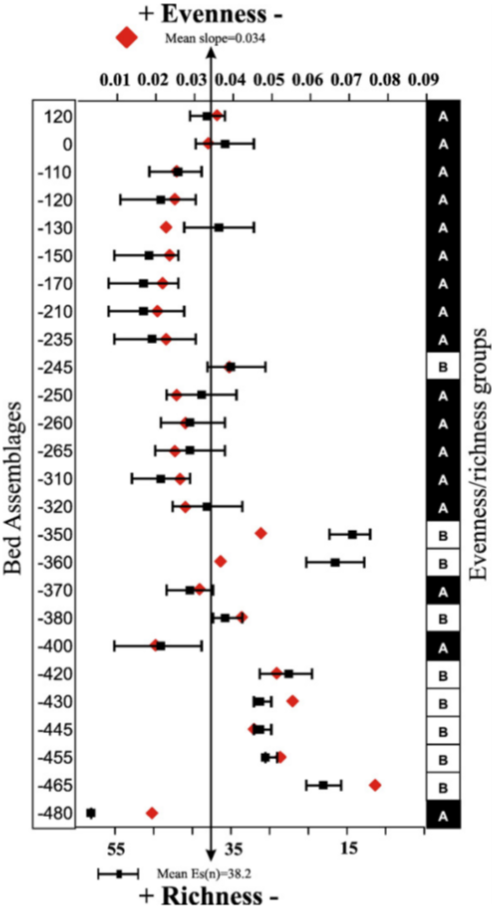
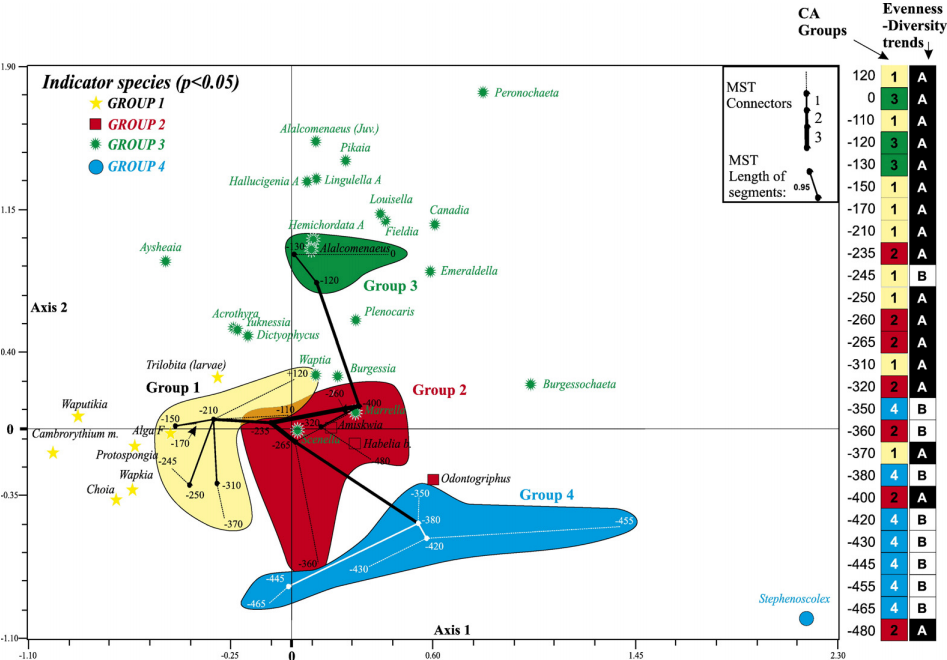
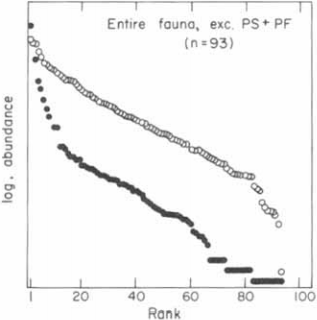
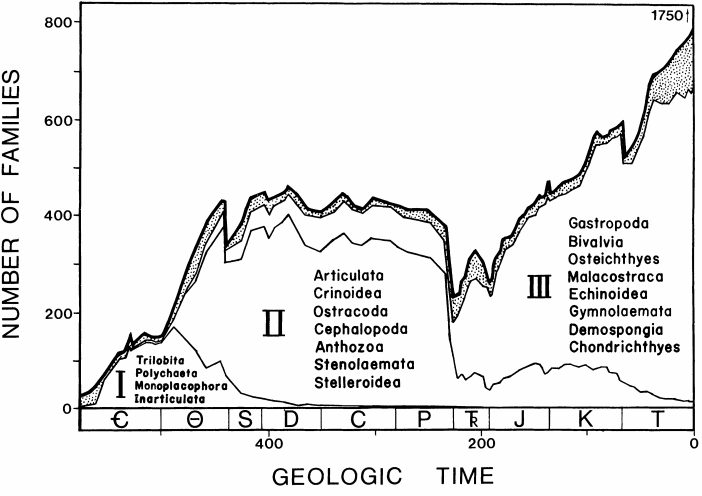
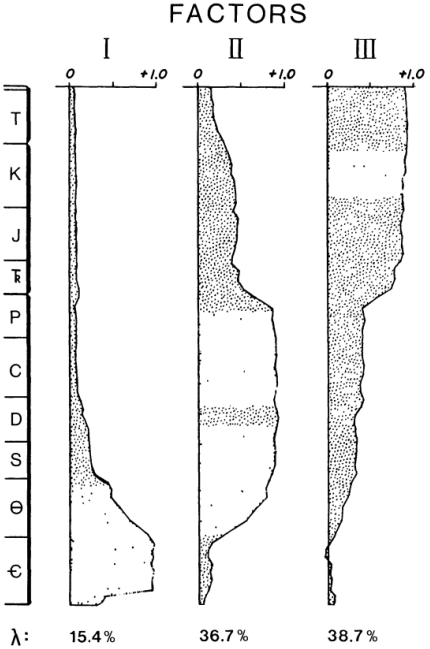
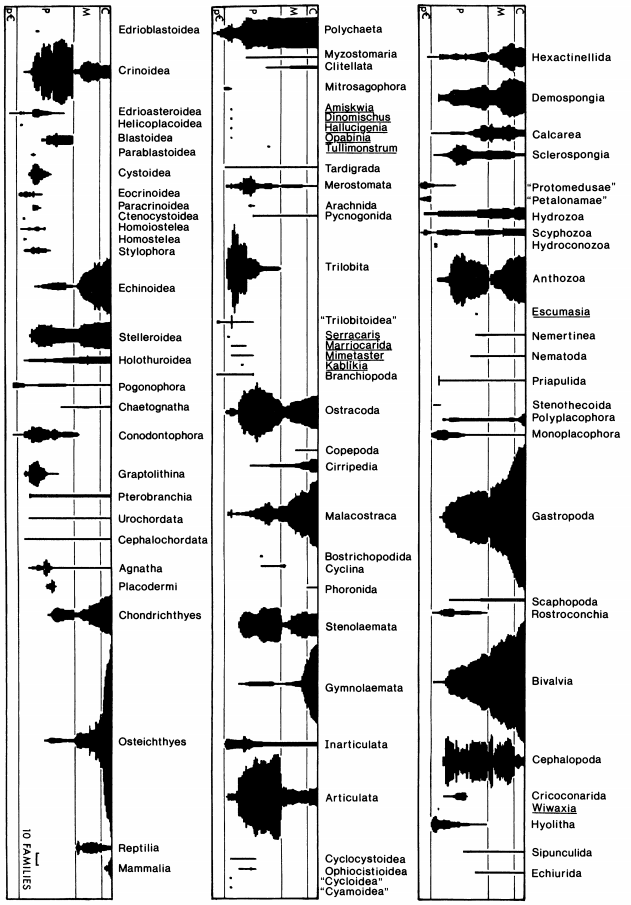
Reconstruct ecosystem characteristics from species count data
Appreciate the strengths and limitations of such data
Evaluate the distinctiveness of Cambrian ecosystems
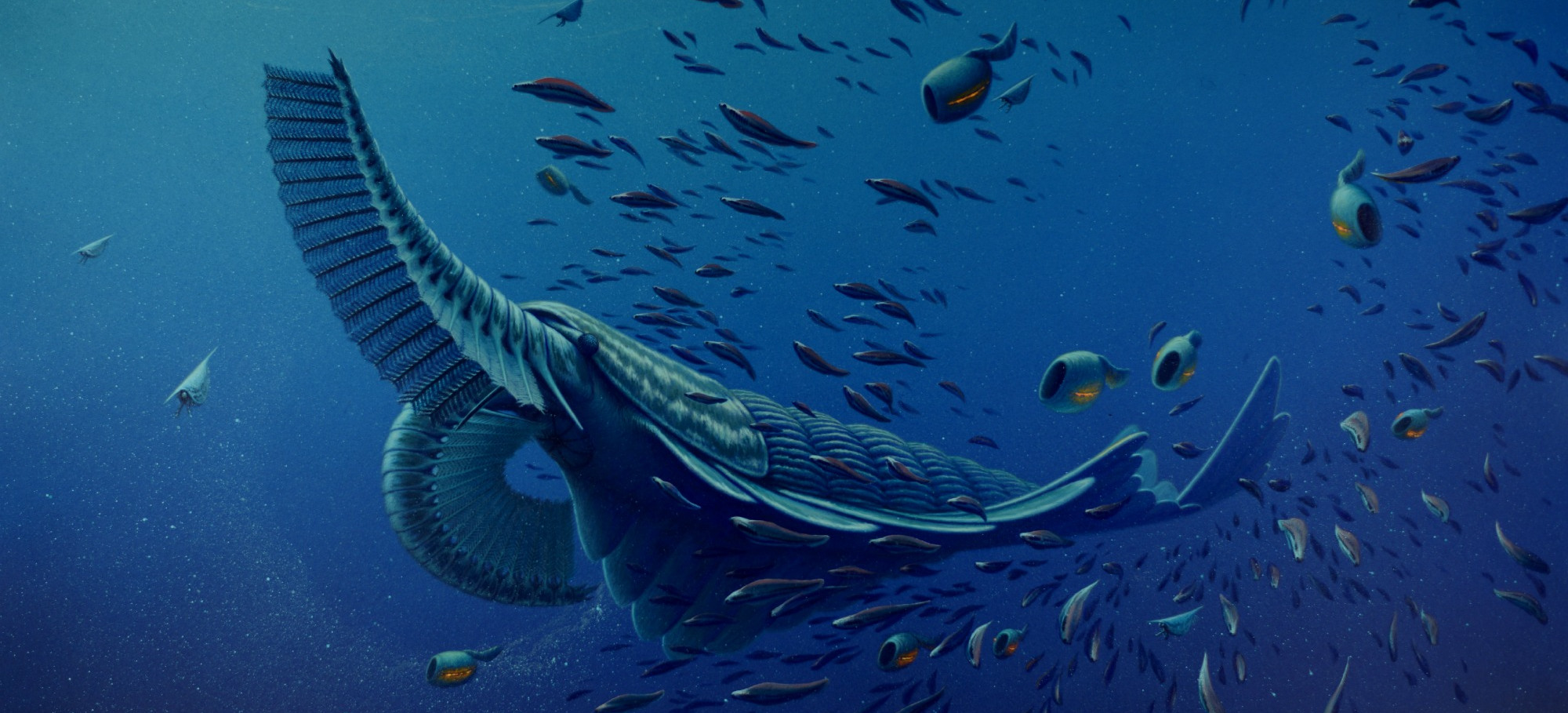

 Systematic sampling allows Caron (foreground) to link samples to specific horizons.
Systematic sampling allows Caron (foreground) to link samples to specific horizons.