
Martin Smith
Laboratory for invertebrate palaeontology and phylogenetics
We are interested in the origins of biodiversity. Our research uses and develops quantitative approaches to reconstruct evolutionary history from the fossil record, with a particular emphasis on the unusual organisms from Burgess Shale-type deposits and their microscopic counterparts, the Small Carbonaceous Fossils.
Timing the pace of the Cambrian Explosion
530 million years ago, the fossil record documents a step change in the complexity and abundance of life on Earth. Our understanding of this ‘Cambrian Explosion’ is fundamentally incomplete. Was it one event or multiple bursts? How did diversification relate to contemporary environmental changes? Can it be explained by present-day evolutionary processes?
Our Leverhulme Trust-funded research is developing mathematical techniques that integrate geological observations from across the globe, generating a single record of biological and environmental changes through time. We are establishing the rate at which animal biodiversity arose, and the triggers and consequences of this riot of evolution.
We have developed StratoBayes, an automated tool for stratigraphic alignment, which will fit independent rock deposits into a single timeline without subjective decision-making. Our new models of morphological evolution will link fossil finds of known ages to points in the tree of life, providing dates and rates of branching points and morphological innovation. Applying our models to detailed datasets of a major animal lineage will link evolutionary progress to environmental change; testing the influence of different factors interpreted as contributors to, or results of, elevated evolutionary rates will establish the feedbacks and interactions between the planet and early animal life.
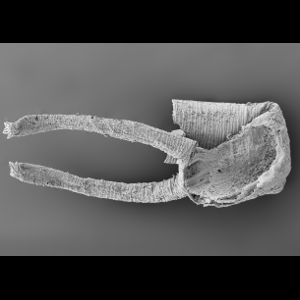
Internal organs in a ‘miracle fossil’
Preternatural preservation of the innards of an ancient ancestral organism give a rare glimpse into the early development of an early euarthropod – a contemporary to the ancestors of krill, crabs and centipedes.
This microscopic fossil, the size of a poppy seed, preserves astonishing details of the brain, blood vessels and nervous system. At half a billion years old, it exposes how early arthropods evolved their distinctive segmented head, and the secrets to their ecological dominance in Cambrian oceans.
Smith, M.R., Long, E.J. et al. (2024). "Organ systems of a Cambrian euarthropod larva". Nature, 632 (8028).
Pioneering bryozoan – or shelly seaweed?
Conventional wisdom holds that all major animal groups trace their origins to the Cambrian period. Our work has reinstated the moss animals (phylum Bryozoan) as the exception to this rule: we have shown that candidate bryozoan fossils from the Cambrian period are actually mineralizing seaweeds.
This pushes back the first occurrence of bryozoans – tentacle-bearing animals that lived in skyscraper-like underwater colonies – by millions of years, to the Ordovician. The delayed appearance of bryozoans suggests that the Cambrian was not as unique a period of innovation as conventionally thought; instead, new body plans continued to be carved out by evolution over a much longer time period.
Yang, J.; Lan, T.; Zhang, X.-G.; Smith, M.R. (2023). "Protomelission is an early dasyclad alga and not a Cambrian bryozoan". Nature, 615 (7952): 468–471.
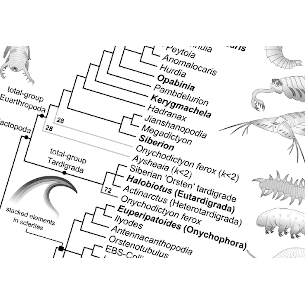
Comparing evolutionary family trees
 M.R. Smith
M.R. Smith
Phylogenetic analysis is the science of reconstructing evolutionary relationships from observations such as morphology and genetic sequences. There are various methods of reconstructing relationships, each with different strengths and weaknesses. My work aims to improve the quality of data that is analysed, and the methods used in analysis. I have developed new ways to compare trees, a first step to testing how well different methods reconstruct true evolutionary patterns; and methods to extract the maximum information from the resulting 'forests' of evolutionary trees.
Smith, M.R. (2022). "Robust analysis of phylogenetic tree space". Systematic Biology, 71 (5): 1255–1270.
Smith, M.R. (2022). "Using information theory to detect rogue taxa and improve consensus trees". Systematic Biology, 71 (5): 1088–1094.
Smith, M.R. (2020). "Information theoretic Generalized Robinson–Foulds metrics for comparing phylogenetic trees". Bioinformatics, 36 (20): 5007–5013.
Brazeau, M.D., Guillerme, T. & Smith, M.R. (2019). "An algorithm for morphological phylogenetic analysis with inapplicable data". Systematic Biology, 68 (4): 619–631.
Smith, M.R. (2019). "Bayesian and parsimony approaches reconstruct informative trees from simulated morphological datasets". Biology Letters, 15:20180632.
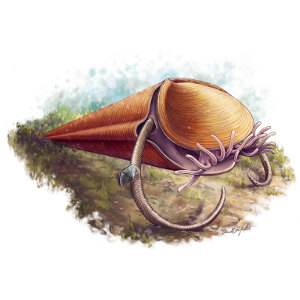
Solving the riddle of the hyoliths
 Danielle Dufault
Danielle Dufault
Hyoliths are one of the most abundant fossils from the Palaeozoic, the era before the dinosaurs. Looking like an ice cream cone with a lid, their identity has long been a mystery: were they aberrant snails, or a long-extinct lineage with no close relatives – a 'failed evolutionary experiment'?
Amazing fossils from North America and China finally solved this 175 year old mystery. Preserving the body, not just the shell, these fossils reveal for the first time a crown of tentacles surrounding the mouth – a distinctive feature that links the fossils to modern brachiopods and horseshoe worms. This shows that Hyoliths lived on the sea floor, plucking food particles from passing water. Settling the affinity of hyoliths sheds new light on the origins of animal body plans during the sudden burst of evolution that is the Cambrian Explosion.
Smith, M.R. (2020). "Finding a home for hyoliths". National Science Review, 7 470–471.
Moysiuk, J., Smith, M.R. & Caron, J-B. (2017). "Hyoliths are Palaeozoic lophophorates". Nature 541: 394–397.
Sun, H.-J., Smith, M.R., Zeng, H., Zhao, F.-Z., Li, G.-X. & Zhu, M.-Y. (2018). "Hyoliths with pedicles illuminate the origin of the brachiopod body plan". Proc. Roy. Soc. B, 285: 20181780.
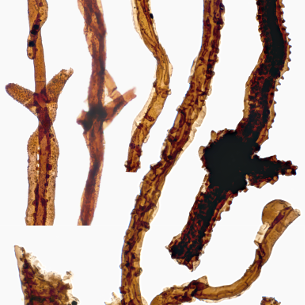
The humble fungus that brought life to land
 M.R. Smith
M.R. Smith
These tiny branching filaments may look unremarkable, but they represent the oldest fossils of terrestrial organisms. Fossils collected in field excursions to Sweden, New York and Scotland allowed us to reconstruct how the hitherto enigmatic organism Tortotubus lived and grew.
These strands represent fragments of extensive subterranean networks, which would have stabilised and nourished the soils in which early plants took root.
Smith, M.R. (2016). "Cord-forming Palaeozoic fungi in terrestrial assemblages". Botanical Journal of the Linnean Society 180 (4): 452–460.
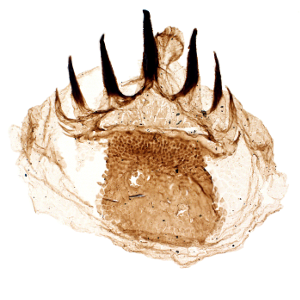
Mini fossils that pack a punch
 T.H.P. Harvey / PalAss
T.H.P. Harvey / PalAss
Small Carbonaceous Fossils (SCFs) are a diverse suite of non-mineralized microfossils representing bits of early organisms. Widespread in space and time, these tiny elements provide an unrivalled view on the rate and tempo of Cambrian evolution. By establishing the identity of a range of problematic SCFs, our research is telling the evolutionary tales locked up in these beautiful fossils.
Smith, M.R., Hughes, G.M.G., Vargas, M.C. & de la Parra, F. (2016). "Sclerites and possible mouthparts of Wiwaxia from the temperate palaeolatitudes of Colombia, South America". Lethaia 49 (3): 393-397.
Smith, M.R., Harvey, T.H.P. & Butterfield, N.J. (2015). "The macro-and micro-fossil record of the Cambrian priapulid Ottoia". Palaeontology 58 (4): 705–721.
Smith, M.R. & Ortega Hernández, J. (2014). "Hallucigenia’s onychophoran-like claws and the case for Tactopoda". Nature 514 (5722): 363–366.
Caron, J.-B., Smith, M.R. & Harvey, T.H.P. (2013). "Beyond the Burgess Shale: Cambrian microfossils track the rise and fall of hallucigeniid lobopodians". Proceedings of the Royal Society B 280 (1767): 20131613.
The 'weird worm' Hallucigenia
 M.R. Smith / Smithsonian
M.R. Smith / Smithsonian
Hallucigenia has always baffled scientists. A 500-million-year-old enigma, this bizarre spiny worm is known from a handful of fossils and just two locations. A study with coauthors Tom Harvey and Jean-Bernard Caron revealed that, in fact, it had relatives all over the world. Whilst examining its defensive spines, we spotted a resemblance with a global family of small spiny fossils: both have a subtle surface ornament and a structure like a stack of ice-cream cones. It seems that quirky Hallucigenia was no recluse: along with its relatives, it formed a cosmopolitan community that spanned the Cambrian seas.
When Hallucigenia was first described in the 1970s, the spines along its back were mistaken for legs, and its head was mistaken for its tail. Now, state-of-the-art electron microscopes have yielded new details from the spines, claws and head of Hallucigenia, exposing its place in life’s evolutionary tree, and how it helps us to reconstruct the common ancestor of all moulting animals.
Smith, M.R. (2017). "Fossil Focus: Hallucigenia and the origin of animal body plans". Palaeontology Online 7 (5): 1–9
Smith, M.R. & Caron, J.-B. (2015). "Hallucigenia’s head and the pharyngeal armature of early ecdysozoans". Nature 523 (7558): 75–78.
Smith, M.R. & Ortega Hernández, J. (2014). "Hallucigenia’s onychophoran-like claws and the case for Tactopoda". Nature 514 (5722): 363–366.
Caron, J.-B., Smith, M.R. & Harvey, T.H.P. (2013). "Beyond the Burgess Shale: Cambrian microfossils track the rise and fall of hallucigeniid lobopodians". Proceedings of the Royal Society B 280 (1767): 20131613.
The 'maybe mollusc' Wiwaxia
The loveable fossil Wiwaxia bears an intimidating coat of scale-mail armour punctuated with lengthy spines. But what is the animal underneath this tough exterior? Could it be a slug, related to the molluscs? Or does it really represent an 'earthworm in disguise'? I've focussed on two clues to assess its affinity. Firstly, its mouthparts have several similarities with the teeth of modern molluscs - and look nothing like worm teeth. Secondly, the animal grows just like some slug-like molluscs that inhabit modern rock pools. However, compelling links with annelid worms suggest that Wiwaxia has a deep evolutionary position and might tell us how both these two body plans became established.
Zhang, Z.-F., Smith, M.R. & Shu, D.-G. (2015). "New reconstruction of the Wiwaxia scleritome, with data from Chengjiang juveniles". Scientific Reports 5: 14810.
Zhao, F.-C., Smith, M.R., Yin, Z.-J., Zeng, H., Hu, S.-X., Li, G.-X. & Zhu, M.-Y. (2015). "First report of Wiwaxia from the Cambrian Chengjiang Lagerstätte". Geological Magazine 152: 378–382.
Yang, J., Smith, M.R., Lan, T, Hou, J.-B., Zhang, X.-G. (2015). "Articulated Wiwaxia from the Cambrian Stage 3 Xiaoshiba Lagerstätte". Scientific Reports 4: 4643.
Smith, M.R. (2014). "Ontogeny, morphology and taxonomy of the soft-bodied Cambrian “mollusc” Wiwaxia", Palaeontology 57 (1): 215-229.
Smith, M.R. (2012). "Mouthparts of the Burgess Shale fossils Odontogriphus and Wiwaxia: implications for the ancestral molluscan radula", Proceedings of the Royal Society B: Biological Sciences 279 (1745): 4287-4295.
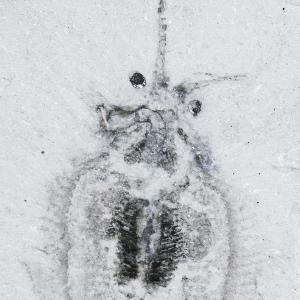
The 'squishy squid' Nectocaris
 M.R. Smith / Nature
M.R. Smith / Nature
From 1911, only a single specimen of Nectocaris was known. But since the 1980s, Royal Ontario Museum collectors have recovered dozens more. This new material transformed our idea of what Nectocaris looked like, and colleague Jean-Bernard Caron and I estabilshed that it resembled a modern-day squid or cuttlefish. This was astonishing: such cephalopods aren't meant to have evolved until much, much later. This could mean that there’s a huge (200 million year) gap in the fossil record, which would turn the conventional concept of cephalopod evolution on its head. Alternatively, Nectocaris may represent an incredible example of convergent evolution - the independent invention of a modern body plan. This question hinges on how far we trust the fossil record; for now, at least, the jury is out.
Smith, M.R. (2020). "An Ordovician nectocaridid hints at an endocochleate origin of Cephalopoda". Journal of Paleontology 94 (1): 64–69.
Smith, M.R. (2013). "Nectocaridid ecology, diversity and affinity: early origin of a cephalopod-like body plan". Paleobiology 39 (2): 345–357.
Smith, M.R. & Caron, J-B. (2011). "Nectocaris and early cephalopod evolution: Reply to Mazurek & Zatoń". Lethaia 44 (4): 369–372.
Smith, M.R. & Caron, J.-B. (2010). "Primitive soft-bodied cephalopods from the Cambrian", Nature 465 (7297): 469–472.
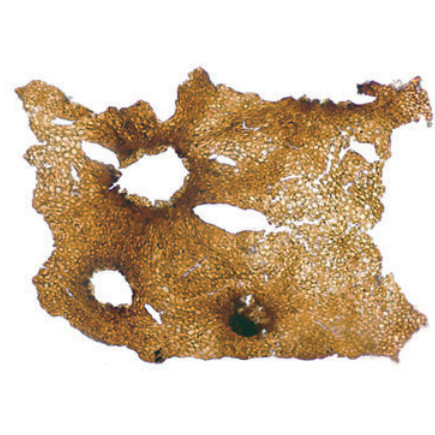
The 'soggy seaweed' Nematothallus
 M.R. Smith / Palaeontology
M.R. Smith / Palaeontology
Tiny fragments of fossilized cuticle, referred to as Nematothallus, have long been implicated in the origin of land plants. Cuticle was an essential adaptation before plants could invade the land – without it, they'd dry up far too quickly. But plants are not the only organisms to have cuticle – so do some seaweeds, lichens, fungi, and animals. How can you tell the difference based on fossil scraps that are less than a millimeter in size?
With co-author Nick Butterfield, I recovered new details of Nematothallus’s reproductive organs, some of which were even preserved with spores attached. Details of these organs allowed our fossils to be reinterpreted as coralline red algae, a type of seaweed that forms parts of coral reefs today. But unlike their modern counterparts, our fossils did not lay down limestone in their skeletons — filling an interesting gap in the evolutionary history of seaweeds.
Smith, M.R. & Butterfield, N.J. (2013). "A new view on Nematothallus: coralline red algae from the Silurian of Gotland". Palaeontology 56 (2): 297-321.
Martin R. Smith
I am interested in the origins of complex animal life, as revealed by the fossil record. Teasing evolutionary information from ancient rocks requires sophisticated mathematical techniques; I develop bioinformatic and phylogenetic methods to better understand the patterns and processes documented by the palaeontological record.
After obtaining an undergraduate degree (BA and MSc) in Natural Sciences from the University of Cambridge, specializing in Earth Sciences and undertaking a Masters’ research project in Palaeobotany with Nick Butterfield, I proceeded to a PhD at the University of Toronto under Jean-Bernard Caron. Based at the Royal Ontario Museum, this was my first encounter with the Burgess Shale fossils that now form a central part of my research portfolio. I subsequently returned to Cambridge to take up a Junior Research Fellowship at Clare College, before moving in 2015 to Durham University, where I am an Associate Professor (i.e. Lecturer) in Palaeontology.

Prof.
Martin R. Smith
SFHEA
Professor in Palaeontology
Department of Earth Sciences
University of Durham,
DH1 3LE
Room ES313
+44 (0) 191 334 2320
martin.smithdurhamacuk
Deng Wang
Deng is interested in the origin and early radiation of Ecdysozoa, with a particular focus on: 1) moulting behaviour and its evolutionary origins; 2) phylogenetic relationships and the timing of divergence among key ecdysozoan lineages, including panarthropods, scalidophorans, and nematoids; 3) the ground pattern (body plan) of ancestral Ecdysozoa; 4) evolution of organ system in Ecdysozoa, such as nervous, musculature, digestive systems and integument. Research materials are significantly based on early Cambrian exceptional fossils (Chengjiang and Kuanchuanpu biotas) and living priapulids.
Deng holds an MSc from Northeastern University, where he studied late Carboniferous coral and sponge reefs, and a PhD from Northwest University and Université Claude Bernard Lyon 1 on ecdysozoan origins and early radiation. He subsequently completed a postdoctoral fellowship at Northwest University.

Dr.
Deng Wang
Research Fellow
+44 (0) 191 334 2347
deng.wangdurhamacuk
Kilian Eichenseer
To reconstruct past interactions between life and the planet, it is necessary to construct a detailed timeline that links evolutionary events with environmental change. This requires the correlation of geochemical and sedimentary observations with an incomplete fossil record.
Kilian is PDRA on a Leverhulme-funded project that will construct Bayesian models of stratigraphy and morphological evolution, which will allow diverse sources of geological data to be integrated into a single timeline, allowing tests of causality and correlation. These models will be applied to the Cambrian Explosion, establishing the character, causes and consequences of this profound evolutionary diversification—thus illuminating the origins of diverse animal-dominated ecosystems and their impact on Earth processes.
Kilian’s background lies in palaeontology and geology, with a focus on quantitative methods and an undergraduate and Master’s degree in Earth Sciences from the University Erlangen-Nuremberg. His Master’s project on Cenozoic reef corals with Wolfgang Kiessling was followed by a PhD on the skeletal mineralogy of marine calcifiers across the Phanerozoic, with Uwe Balthasar at the University of Plymouth. Further research interests include the temporal scaling and spatial distribution of palaeoclimate data, and long-term trends in the prevalence of biotic and abiotic factors in macroevolution.

Dr.
Kilian Eichenseer
Postdoctoral research associate
+44 (0) 191 334 2347
kilian.eichenseerdurhamacuk
Alex Butryn
Alex has an interdisciplinary background, with an undergraduate degree in Natural Sciences and an MPhil in Computational Biology. His Master’s project with Roger Benson used “realistic simulations” to investigate the effect of missing data on phylogenetic inference. These experiences were crucial in developing research skills and shaping his academic interests, which revolve around palaeontology, phylogenetics and applied mathematics. Upon completing his Master’s, Alex worked as a tutor, education consultant and business analyst, before returning to academia.
Alex's Leverhulme-funded PhD research combines evolutionary theory, statistical methods and data from the fossil record to contribute to the understanding and evaluation of the evolutionary interrelationships of extinct taxa.

Mr.
Alexander Butryn
Postgraduate student
a.butryn2newcastleacuk
Co-investigators
Our Leverhulme-funded research project exploring the pace of the Cambrian Explosion is a collaborative effort with Tom Nye (Statistics, Newcastle) and Andrew Millard (Archaeology, Durham).
Alumni
Previous group members include:
- Alavya Dhungana
- Heda Agić
- Matthias Sinnesael (now Trinity College Dublin)
- Emma Long (now University of Exeter)
- Stella Felsinger (now University of Oxford): Untangling the tree of life: Which partitioning strategies improve phylogenetic inference?
Masters by Research
We welcome applications from undergraduate students looking to undertake MScR training.
Applications are due in early April, with interviews soon thereafter. Applicants will automatically be considered for a Stephen Mills Scholarship, which covers tuition fees at the home student rate. We may be able to consider self-funded applications received after this date.
We welcome enquiries or project proposals from potential applicants, who should be on track to receive a strong Bachelor's degree. Overseas applicants may be eligible for Erasmus, Fullbright, Chevening or other scholarships. Durham University also offer means-tested scholarships, and maintains lists of external scholarship opportunities and other funding sources.
For more information, please contact martin.smithdurhamacuk.

Potential projects
Do clades exhibit an early burst of disparity?
It has often been claimed that major groups are characterized by an early burst of disparity (e.g. Hughes et al. 2013; Oyston et al. 2015). Extrinsic and intrinsic factors have been proposed, but no satisfactory explanation is forthcoming.
In the context of recent work by Budd and Mann, we suggest that two null models may offer a satisfactory account for this claim:
- High early disparity is a prerequisite for becoming a ‘major clade’;
- High early disparity is an artefact of using phylogenetic datasets, which do not contain autapomorphies.
This project will use the software REvoSim to simulate evolutionary data in order to compare observed patterns of disparity to expected values under unconstrained evolutionary change. This will make it possible to test whether 'early bursts' of evolution represent an authentic feature that requires a macroevolutionary explanation, or whether they represent an necessary prerequisite for survival to the present and thus an emergent feature of natural selection in the presence of extinction.
Finding a home for a Carboniferous oddity
Typhloesus is a bizarre swimming animal from the Carboniferous Mazon Creek Lagerstätte, recently reinterpreted as a swimming mollusc. This project will undertake a phylogenetic reappraisal of this affinity, testing the proposed gastropod affinity against other positions within Mollsuca, and evaluating the degree of convergence and the implications for molluscan evolution.
Reading: Conway Morris, S. & Caron, J.-B. A possible home for a bizarre Carboniferous animal: is Typhloesus a pelagic gastropod?: Biology Letters, v. 18, 2022.0179.
Why do Cambrian organisms lack mineralization?
The Ediacaran–Ordovician transition sees a profound shift in the role and use of mineralization within organisms. This project aims to test the hypothesis that mineralization intensity corresponds to predation pressure, and, in turn, primary productivity. This provides a possible explanation for the increase in mineralization through the Cambrian explosion, and the selective nature of mineralization in the Ediacaran – with high productivity settings dominated by highly mineralized cloudinid-dominated reefs, in contrast to the non-mineralized nature of deep-water vendobionts.
Using a combination of extant and fossil datasets, it will seek to establish three relationships that, if true, would support the hypothesis:
- Is the cost of mineralization (or not mineralizing) related the probability of predation?
- Is the abundance of predators a good proxy for the probability of predation?
- Does (primary) productivity predict the abundance of predators?
Reading: Wood, R., and Zhuravlev, A. Yu., 2012, Escalation and ecological selectively of mineralogy in the Cambrian Radiation of skeletons: Earth-Science Reviews, v. 115, p. 249–261.

An improved Bayesian framework for global stratigraphic correlation
We have developed a Bayesian framework for stratigraphic correlation based on δ13Corg, U/Pb dates and biostratigraphy. We are looking for MScR and PhD students to develop additional modules that will allow a broader suite of stratigraphic information to be incorporated into this model. Possible focal areas include Strontium isotope data; integration of hiatuses; incorporation of the fossil record; and integration of divergent preservational modes.
All the better to see you with: controls on visual acuity in Cambrian communities
The evolution of vision offered significant advantages to the earliest organisms to bear eyes. Simple physical considerations allow visual acuity to be accurately reconstructed from suitably preserved fossils. This project will constrain the optical capabilities of soft-bodied fossils from Cambrian Lagerstätten, using measurements obtained from published specimens. Visual acuity will be linked to environmental factors to establish the primary evolutionary forces controlling vision in the Cambrian, with a view to better constraining the ecology and environmental parameters of early animal ecosystems.

How does morphology evolve?
Understanding the biological processes by which observable phenotypic evolution occurs is a fundamental goal of macroevolutionary study – and modelling morphological evolution is key to reconstructing the relationships and evolutionary history of life.
This project will test a series of hypotheses of how morphological variety originates by identifying patterns of evolution in a collection of hundreds of existing morphological datasets from a wide range of vertebrate, invertebrate and non-metazoan groups. The student will establish whether widespread tenets of palaeontological phylogenetics (e.g. equal weights parsimony) can truly account for observed morphological diversity, improving our understanding of how evolutionary processes lead to morphological novelty. This in turn will improve models of phylogenetic inference, and our understanding of evolutionary history.
Reliability of phylogenetic results
Much of modern biology is underpinned by frameworks of relationships arising through phylogenetic analysis. As such, it is more important than ever to be able to distinguish results that are supported by strong evidence from those likely to be overturned as new data accumulate.
Hillis and Huelsenbeck (1992) propose the skewness of tree lengths as a proxy for the reliability of phylogenetic results obtained from a dataset. This project will implement this reliability measure and apply it to palaeontological data matrices in order to resolve disagreements between competing phylogenetic studies and to establish the relative contributions of fossil and extant taxa to phylogenetic reconstructions.
PhD
We are currently looking to recruit excellent students to our postgraduate research programmes.
A diverse range of projects are available, based on independent fieldwork, museum collections, and laboratory-based data analysis.

The most popular routes to PhDs in the Smith lab are:
- RCUK-funded projects (home students): these are advertised each autumn through the NERC Iapetus doctoral training program; applications are due by early January (assessment criteria). If you'd like to propose your own project, get in touch over the summer.
- Durham Doctoral Studentships: these competitive positions allow a home or overseas student to take up a project advertised under the RCUK umbrella, or one of their own devising. Applicants are advised to contact potential supervisors well in advance of the mid-January deadline.
- Personal scholarships: many alternative funding sources are available, which may be tied to an advertised project or to a project devised jointly with a potential supervisor – feel free to get in touch. Durham maintain lists of scholarship opportunities and other funding sources.
Potential projects
Convergence or cryptic evolution? The origins of the cephalopod body plan
500 million years ago, life on earth was fundamentally transformed by the geologically rapid emergence of complex animal-dominated ecosystems. This ‘Cambrian Explosion’ permanently altered the dynamics of biology and geology on a planetary scale – but what evolutionary processes led to the sudden dominance of macroscopic organisms, and their concomitant interactions with the oceans, sediment and biosphere?
Cephalopods – a charismatic group of molluscs that includes cuttlefish, octopus, and the extinct ammonites and belemnites – offer an illuminating perspective on the dynamics of Cambrian evolution. The conventional view interprets a suite of snail-like Cambrian fossils as representing the gradually evolving roots of the cephalopod lineage. This view has recently been challenged by Nectocaris, an early Cambrian fossil that strikingly resembles a modern squid. If Nectocaris is a cephalopod, then cephalopods evolved from a non-mineralized ancestor in the height of the Cambrian explosion, with an early evolutionary history that largely escaped the fossil record.
This controversial interpretation supports an interpretation that sees anatomical blueprints of the major animal lineages becoming ‘fixed’ in the earliest Cambrian. Reconstructing the ancestral cephalopod as internally shelled, physiologically energetic, and jet propelled is at odds with the conventional viewpoint of evolutionary ‘progress’ from sluggish forebears to ‘advanced’ modern taxa.
If Nectocaris is not a cephalopod, however, then modern squid have ‘reinvented’ the blueprint established by Nectocaris. This remarkable degree of convergence implies that physical processes constrain biological possibility, with evolution only able to arrive at a finite number of discrete ‘body plans’.
This PhD proposal will conduct the first quantitative test of the relationships of Nectocaris. This objective evaluation will illuminate its implications for the nature of evolutionary innovation.

An improved Bayesian framework for global stratigraphic correlation
We have developed a Bayesian framework for stratigraphic correlation based on δ13Corg, U/Pb dates and biostratigraphy. We are looking for MScR and PhD students to develop additional modules that will allow a broader suite of stratigraphic information to be incorporated into this model. Possible focal areas include Strontium isotope data; integration of hiatuses; incorporation of the fossil record; and integration of divergent preservational modes.
Postdoctoral Fellowships
We invite potential postdoctoral fellows with compatible research interests to join the Palaeoecosystems group, and welcome the opportunity to support any potential applications. Besides national and international schemes (e.g. NERC/Leverhulme/Royal Society), Durham University funds its own Addison Wheeler Fellowships.

Undergraduate Research
A range of undergraduate-level research projects are available for Durham and non-Durham students who wish to gain palaeontological experience. Bursaries may be available from the Palaeontological Association (Deadline: late February).
As a Senior Fellow of the Higher Education Academy, I apply evidence-based pedagogical theory to develop an engaging, balanced and outcome-directed teaching approach.

I currently lead palaeobiology teachings across the undergraduate curriculum, and supervise third- and fourth-year research projects.
- Level 1 practical exercise (with notes): research-led investigation of Ediacaran organisms.
- Level 2: Palaeoecosystems
- Level 2: Frontiers in Palaeobiology
- Level 3: Habitable Environments (Astrobiology)
We have produced a variety of software packages to facilitate phylogenetic analysis and the interpretation of results.
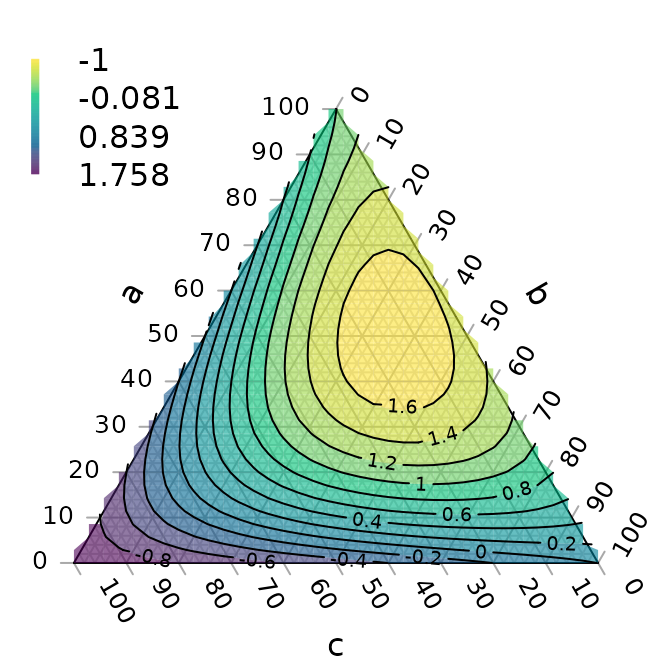
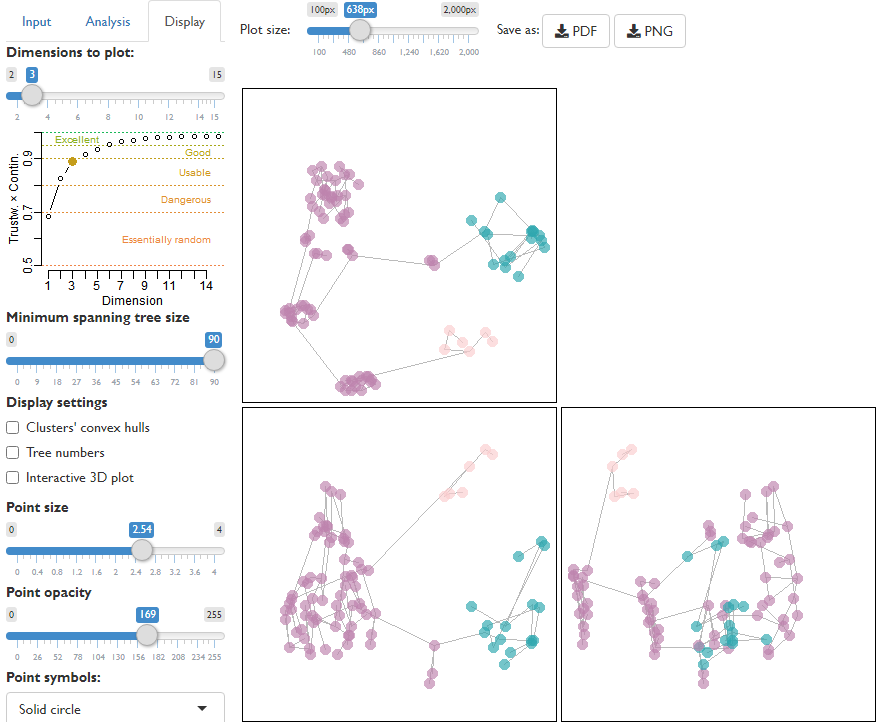
MapTrees: interactive user interface for the creation, visualization and evaluation of tree landscapes.
TreeTools: utilities for the creation, modification and analysis of phylogenetic trees.
TreeSearch: phylogenetic tree search in R, with an appropriate treatment of ‘inapplicable’ characters.
Rogue: Identify rogue taxa to improve consensus trees.
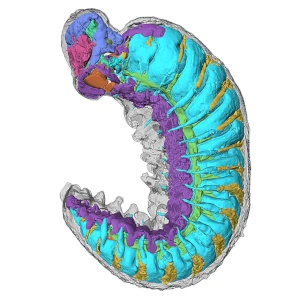
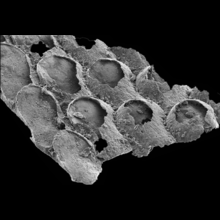
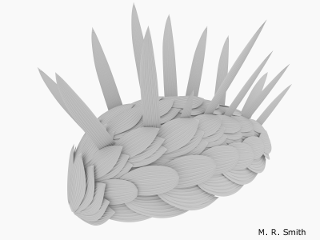
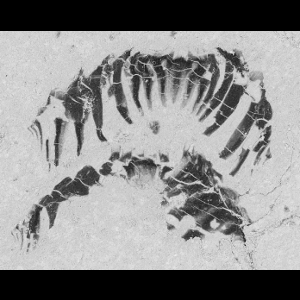 M.R. Smith / Royal Society
M.R. Smith / Royal Society